Before initial administration into humans and prior to receiving regulatory approval, pharmaceutical and biotechnology companies must show evidence of a compound’s biological activity and provide data indicating that the drug is reasonably safe.
Iris Pharma offers a safety assessment program that meets GLP standards, including acute local toxicity, chronic local toxicity, maximum tolerated dose (MTD), corneal anesthesia, and ocular histopathology assessment. Also, depending on the properties of the product, tests for measuring lacrimation and pupil reactivity can be included as well.
Iris Pharma quantifies acute local toxicity using the following:
Guidelines: OECD guideline for the testing of chemicals - Test No. 405 Acute Eye Irritation/Corrosion (adopted 9 October 2017 - corrected 26 June 2020)
Administration: Topical ocular administration: 1 x 100 µl
Animals: Albino rabbits: 3 per group
Evaluation: Draize test at 5 min, 60 min, 1, 2 and 3 days (4 and 7 days if necessary)
Groups: Test article, vehicle (when applicable), control substance
Iris Pharma quantifies chronic local toxicity using the following:
Guidelines: OECD 410: Repeated Dose Dermal Toxicity: 21/28-Day study, adopted 12 May 1981. European Commission Directive # 92/69/EC (July 31, 1992) - Offic. J. Eur. Commun. 1992; 35 L383: B9
Formulation (GMP): Eyedrop, gel, ointment, devices (insert, implant…), particles, patch, cream...
Administration: Topical ocular application, intravitreal injection (IVT), subconjunctival injection, subTenon injection (SBT), retrobulbar injection, periocular injection, intracameral injection, subretinal, intrascleral, transscleral, intrastromal, iontophoresis…
Animals: Albino rabbits: at least 5 males and 5 females per group
Dosage: Multiple daily administration for 28 days or more
Evaluation:
Groups: Test article, vehicle (when applicable), control substance, reference substance (when applicable).
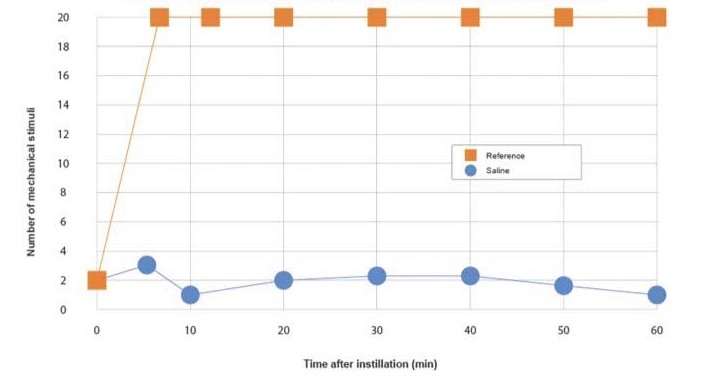
Iris Pharma performs corneal anesthesia tests, including:
Guideline: Guideline on non-clinical local tolerance testing of medicinalproducts - EMA/CHMP/SWP/2145/2000 Rev. 1, Corr. 1
Formulation: Eyedrop, gel, ointment, cream, …
Administration: Topical, iontophoresis…
Animals: Albino rabbits
Evaluation: Cochet Bonnet esthesiometer
Parameters: Number of mechanical stimuli necessary to induce a blinking reflex within 1 hour
Reference substances: Oxybuprocaine / tetracaine
Graph legend: Corneal anesthesia evaluated by the number of mechanical stimuli necessary to induced the blinking reflex
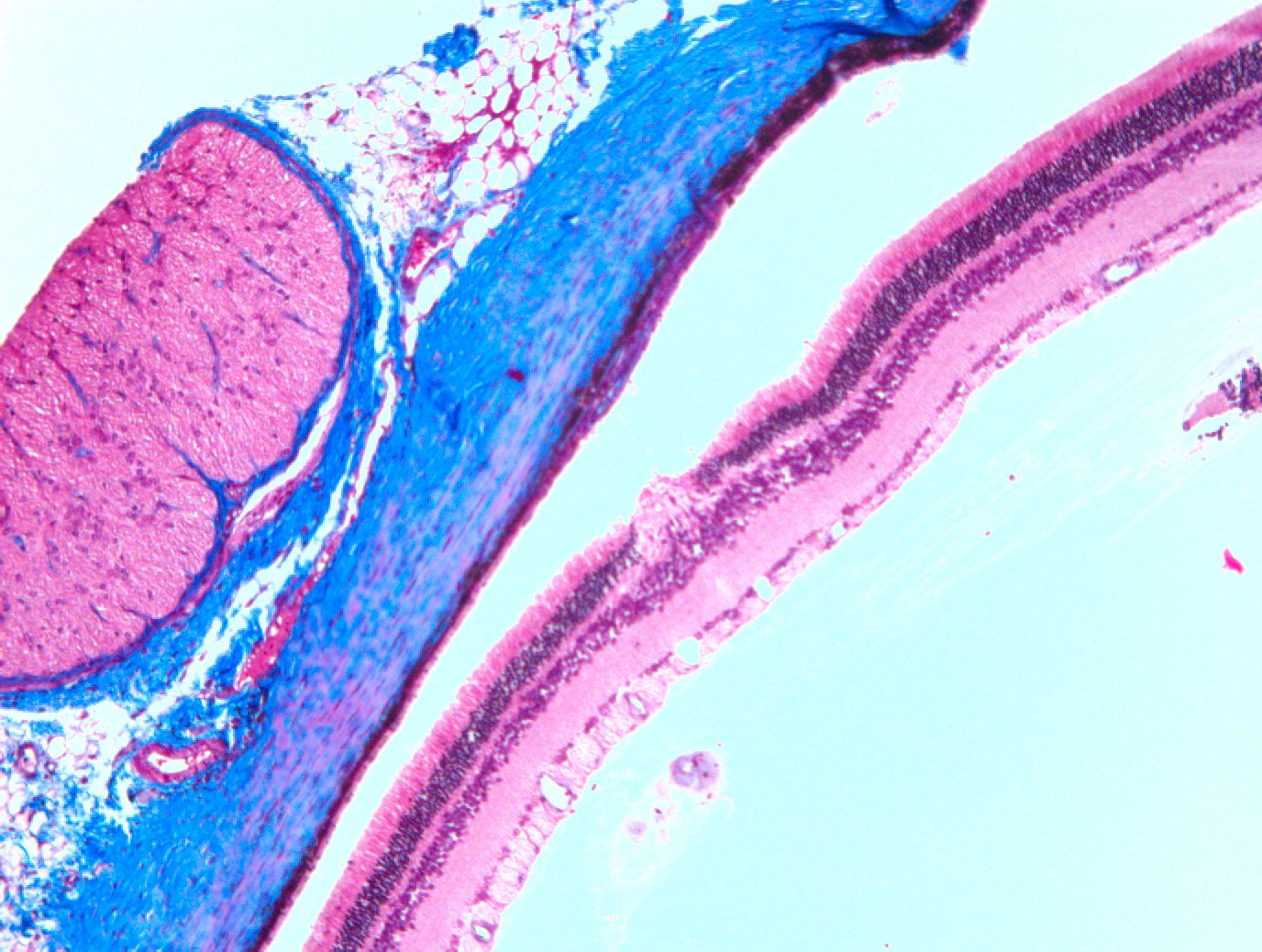
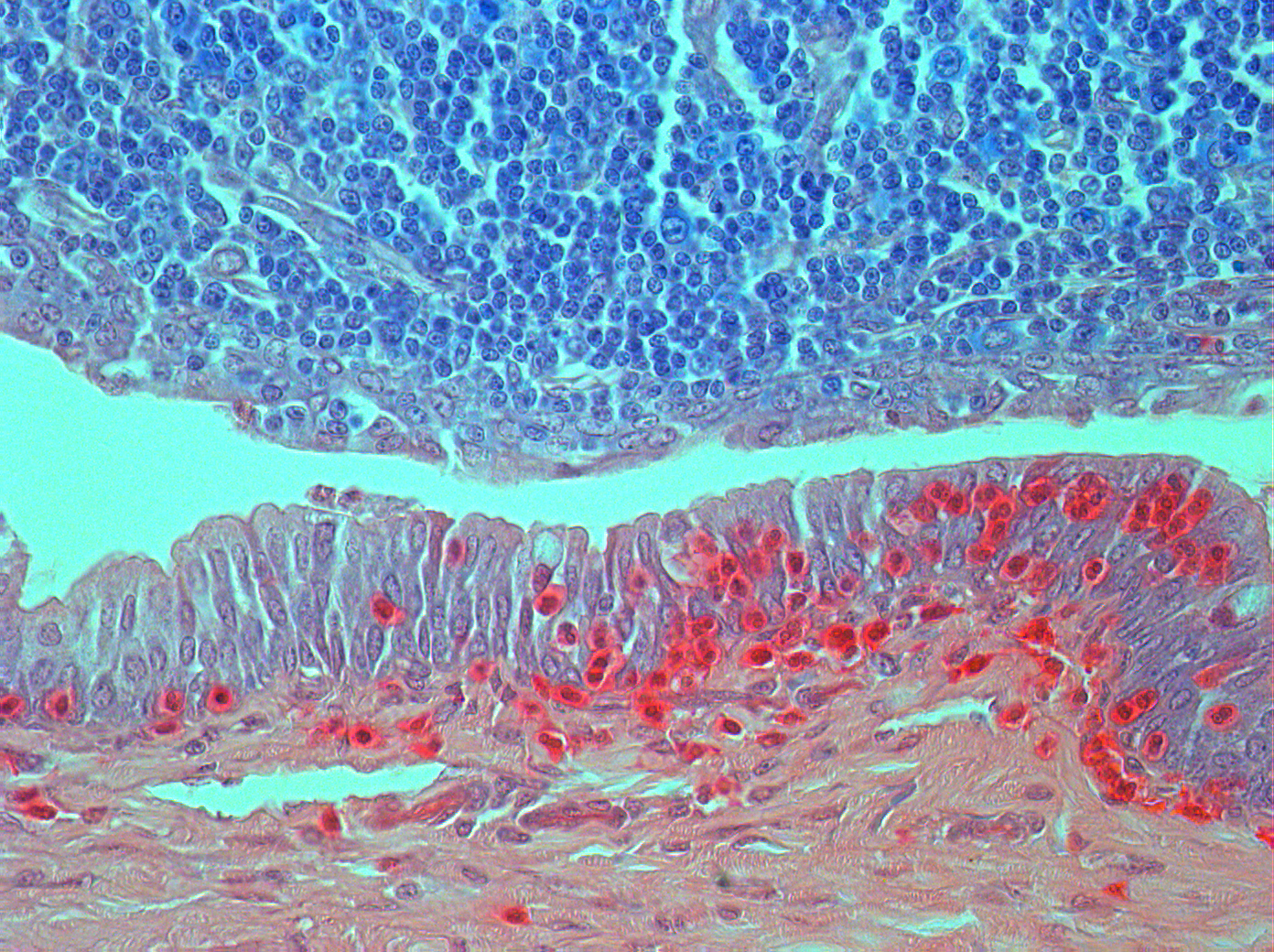
The performance of ocular histopathology is a true craft, requiring highly trained and specialized staff. The difference in density from one ocular structure to another may go unnoticed by those who have little experience in this field.
Iris Pharma’s team has been working for more than 30 years on detailing and analyzing every structure of the eye, even going so far as to observe inflammatory cells in the vitreous body and aqueous humor. Due to these skills, our pathologists are able to use several techniques and perform specialized evaluations, including:
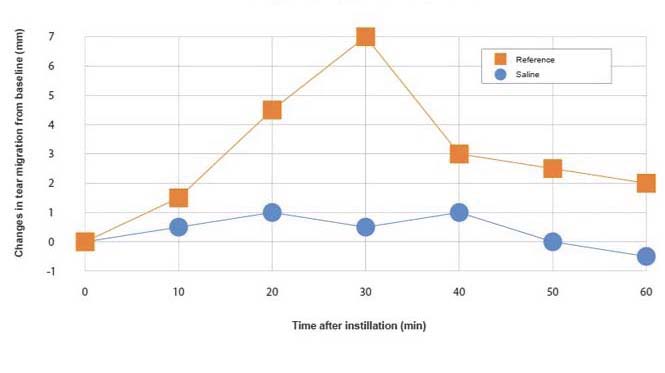
Guideline: Guideline on non-clinical local tolerance testing of medicinalproducts - EMA/CHMP/SWP/2145/2000 Rev. 1, Corr. 1
Administration: Topical, iontophoresis...
Animals: Albino rabbits
Evaluation: Schirmer strips, microcapillaries
Parameters: Quantity of tears collected in 5 min
Reference substances: Pilocarpine
Graph legend: Changes in tear migration after one instillation
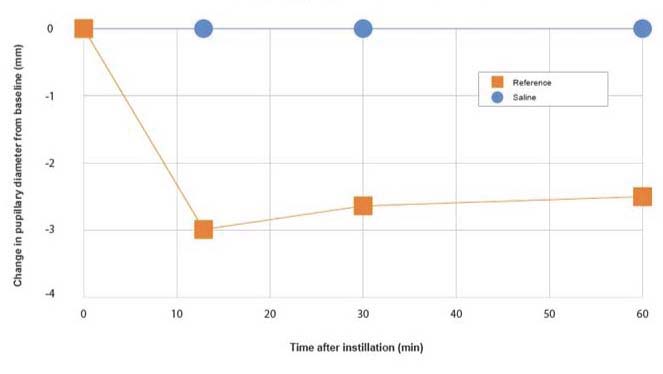
Guideline: Guideline on non-clinical local tolerance testing of medicinalproducts - EMA/CHMP/SWP/2145/2000 Rev. 1, Corr. 1
Administration: Topical, iontophoresis…
Animals: Albino rabbits
Evaluation: Graduated rule
Parameters: Measurement in mm versus time
Reference substances: Pilocarpine or atropine
Graph legend: Changes in pupillary diameter after one instillation